Accessories and Consumables
MRI Information
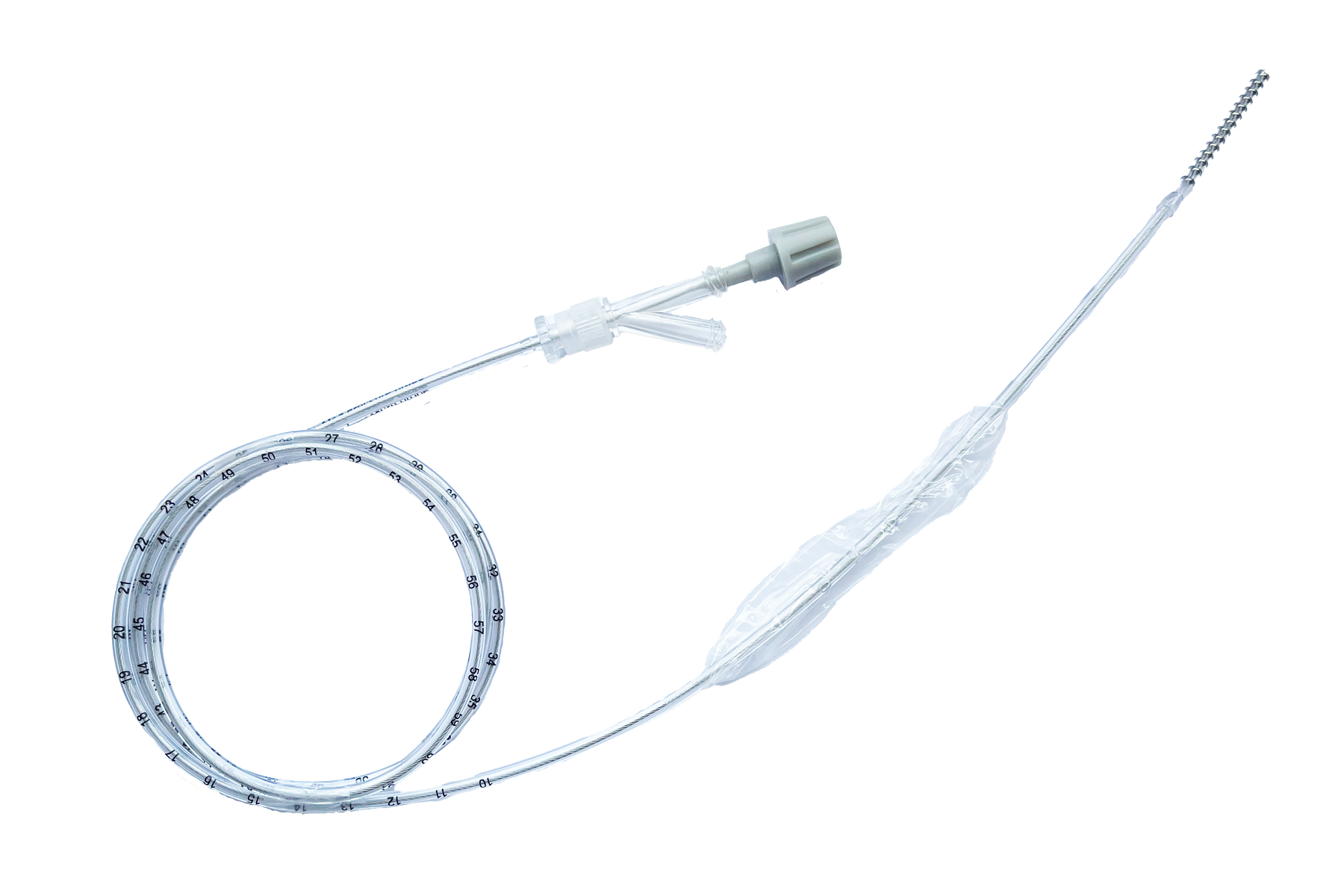
The FluxMed BA-A-008 Esophageal Probe is MRI conditional. This means that a patient using it may be safely scanned under certain conditions. Failure to follow these conditions may result in an injury.
Products that are intended to be used in a MR environment are classified into three categories, as
defined by the ASTM International standard F2503-13:
- MR Safe: a medical device that poses no known hazards resulting from exposure to any MR environment. MR Safe medical devices are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic
- MRI Conditional: a medical device with demonstrated safety in the MR environment within defined conditions including conditions for the static magnetic field, the time-varying gradient magnetic fields, and the radiofrequency fields
- MR Unsafe: a medical device which poses unacceptable risks to the patient, medical staff or other persons within the MR environment
Before going into the MRI room, screen patients for implants, devices, and other metallic objects. Assume anything unknown is MR Unsafe.
Screen objects to ensure that anything entering the scan room is MR Conditional or MR Safe. Match conditions on MR Conditional devices with your scanner. All metals, even non-ferromagnetic ones, have the potential to heat up and cause burns. Look for these symbols on the medical equipment's labels:

You will find the MR Conditional symbol on the label of the FluxMed Esophageal Probe. The product was submitted to the following tests:
- ASTM F2052-21: Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Device in the Magnetic Resonance Environment
- ASTM 2213-17: Standard test method for measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment
- ASTM 2182-19e2: Standard Test method for measurement of RF Induced Heating on or Near Passive Implants during MRI
To safely scan a patient with the probe, make sure to follow the conditions detailed below:
| Parameter | Conditions of use |
|---|---|
| Static Magnetic Field Strength (Bₒ) | 1.5T, 3.0T |
| Maximum Spatial Field Gradient (SPG) | 20 T/m and 2,000 gauss/cm |
| RF Polarization | Circularly Polarized (CP) |
| RF Transmit Coil Type | Any Transmit RF Coil may be used |
| MR System (RF) operating Modes or Constraints | Normal Mode |
| Whole-Body Averaged SAR | Whole-Body Averaged SAR ≤ 2 W/kg |
| Scan Duration and Wait Time | Scan for 15 minutes of continuous RF exposure with one or more MR imaging pulse sequences (scans or series) without cooling period. |
| Artifacts | The presence of the probe may produce an image artifact. |
| Additional information | The health state of the patient or the presence of other implants may require a reduction of the MRI limits. The stylet/guide wire must be removed prior to entering the MRI room. No other implant should be present within 3 cm of Pressure Probe BA-A-008 during MRI scanning. |



